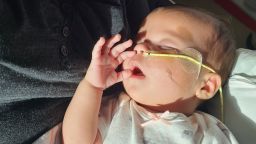
Consumers are at risk as recalled products linger on store shelves, a situation highlighted by a recent discovery of ByHeart powdered infant formula still available for purchase. This alarming finding occurred in a Kroger store just weeks after a nationwide recall was issued on November 11, 2023, due to potential contamination linked to infant botulism cases.
A shopper reported seeing cans of the recalled formula with a notice from the November recall still taped underneath. The incident drew immediate attention from food safety experts, including attorney Bill Marler, who is currently representing families affected by the contaminated product. Marler stated, “This is nuts,” reflecting widespread concern about the safety measures taken by retailers.
In response to this incident, Kroger’s press office asserted that the company had removed the affected product and implemented a block at the point of sale to prevent any sales of the recalled items. Despite these claims, the company did not clarify why the formula was still on the shelf. The U.S. Food and Drug Administration (FDA) has also been alerted about the situation through its consumer complaint portal.
On December 12, 2023, the FDA issued warning letters to multiple retailers, including Kroger, Target, Albertsons, and Walmart, after inspectors reported finding cans of ByHeart for sale in stores across 36 states, despite the recall. The problem of recalled products remaining on shelves is not new. In 2022, the Consumer Product Safety Commission (CPSC) imposed a civil penalty of $13 million on TJX, the parent company of TJ Maxx and Marshalls, for selling over 1,200 units of recalled items, including dangerous infant sleepers.
Peter Feldman, acting chairman of the CPSC, emphasized the seriousness of the issue, stating, “When CPSC recalls a product, it becomes illegal to sell that product.” Retailers sometimes struggle to communicate recall information effectively to all employees, leading to lapses in compliance. Implementing sales blocks, like those claimed by Kroger, can help prevent consumers from purchasing recalled products.
Despite the presence of regulatory bodies, the effectiveness of recall systems is often challenged. The CPSC and FDA conduct checks to ensure recalled products do not remain available, but these checks are not uniform across all situations. The CPSC has ramped up its efforts, with its ESAFE team issuing 33,000 takedown orders for recalled products online in the last three months, marking a significant increase compared to previous years.
Infant products and electronics are particularly scrutinized due to their potential risks. Feldman advises consumers to check the CPSC website for recalls before purchasing secondhand items, especially baby products that might still be in circulation long after a recall has been issued.
Recent trends indicate an increase in recalls, with the CPSC reporting 357 recalls in fiscal year 2025, up from 238 in 2020. This rise highlights the challenges faced by the government in monitoring compliance effectively. Frank Yiannis, a former FDA deputy commissioner, pointed out that products such as infant formula require vigilant oversight.
The ByHeart recall was triggered after an investigation revealed a concerning number of infant botulism cases associated with the formula. Testing confirmed the presence of botulinum spores in several samples, leading to the recall of all lots of ByHeart Whole Nutrition powdered infant formula. As of December 17, 2023, 51 infants across 19 states have been affected, with all requiring hospitalization. Fortunately, there have been no fatalities, largely due to the availability of Baby Botulism Immune Globulin, a treatment that helps infants combat the toxin.
Despite the efficacy of treatments, the costs and potential long-term effects on affected infants remain significant concerns. Yiannis criticized the slow response from both ByHeart and regulatory agencies, indicating that confusion surrounding the recall could have contributed to its persistence on store shelves.
ByHeart has since paused production and is conducting an audit of its supply chain to identify potential contamination sources. The company issued a public apology, recognizing the distress caused by the recall and urging parents to monitor their infants for symptoms of botulism.
As the case of ByHeart illustrates, the failure to swiftly remove recalled products from shelves can have serious implications for consumer safety. Advocacy groups like Stop Foodborne Illness stress the importance of accountability among companies to prevent such situations from occurring.