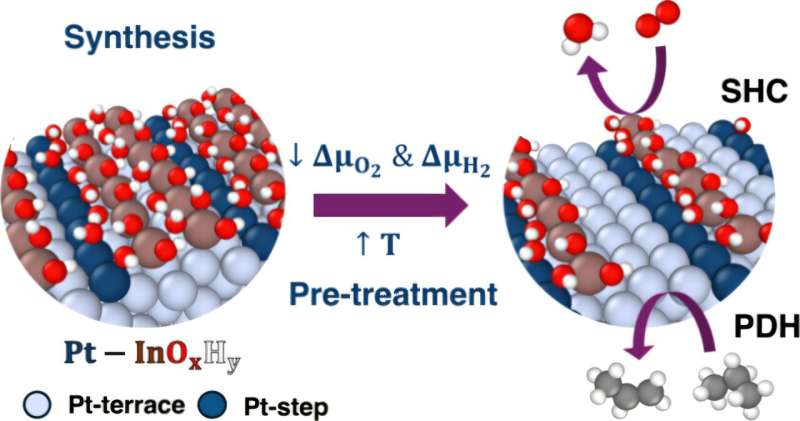
Countless everyday products, from plastic containers to outdoor furniture, rely on a crucial chemical transformation: converting propane into propylene. Research from the University of Rochester has recently advanced understanding of this process through the development of algorithms that reveal atomic-level details of this complex chemistry. Published in the Journal of the American Chemical Society on November 13, 2025, the study sheds light on how nanoscale catalysts facilitate this transformation.
In a significant breakthrough, a previous study in 2021 demonstrated that chemists could streamline the conversion of propane to propylene. By utilizing tandem nanoscale catalysts, researchers integrated multiple reaction steps into a single process. This innovation promised to enhance yield and reduce costs for manufacturers. However, the atomic mechanisms underlying this transformation remained unclear, limiting the broader application of the technique in other industrial contexts.
Algorithms Illuminate Complex Catalytic Reactions
The research team at the University of Rochester, led by Siddharth Deshpande, an assistant professor in the Department of Chemical and Sustainability Engineering, created algorithms designed to analyze the intricate interactions occurring within these nanoscale catalysts. “There are so many different possibilities of what’s happening at the catalytic active sites, so we need an algorithmic approach to very easily yet logically screen through the large amount of possibilities that exist and focus on the most important ones,” Deshpande explained.
Deshpande and his Ph.D. student, Snehitha Srirangam, discovered unexpected findings during their detailed analysis. Notably, the oxide that plays a critical role in the catalytic reaction exhibited a preference for forming around defective metal sites. This selective growth was essential for the catalyst’s stability. Furthermore, despite varying chemical compositions, the oxide consistently maintained its association with these defective sites, which underpins its functional role.
The implications of this research extend beyond propane conversion. Deshpande emphasized that the insights gained from their algorithms could enhance the understanding of other significant chemical reactions, including methanol synthesis, which is vital for producing a variety of products, from paints to fuel cells.
Future Applications and Benefits
Deshpande envisions that this research could lead to more strategic approaches for companies seeking efficient methods to produce propylene and other industrial materials. By moving away from traditional trial-and-error techniques, firms could leverage the knowledge gained from these algorithms to enhance productivity and reduce costs.
“Our approach is very general and can open the doors to understanding many of these processes that have remained an enigma for decades,” Deshpande stated. “We know these processes work, and we produce tons of these chemicals, but we have much to learn about why exactly they’re working.”
With this breakthrough, the University of Rochester contributes significantly to the ongoing evolution of chemical engineering, providing tools that may streamline production processes and foster innovation in material sciences. The research underscores the importance of integrating computational methods to enhance our understanding of complex chemical systems, potentially revolutionizing how industries approach chemical production in the future.