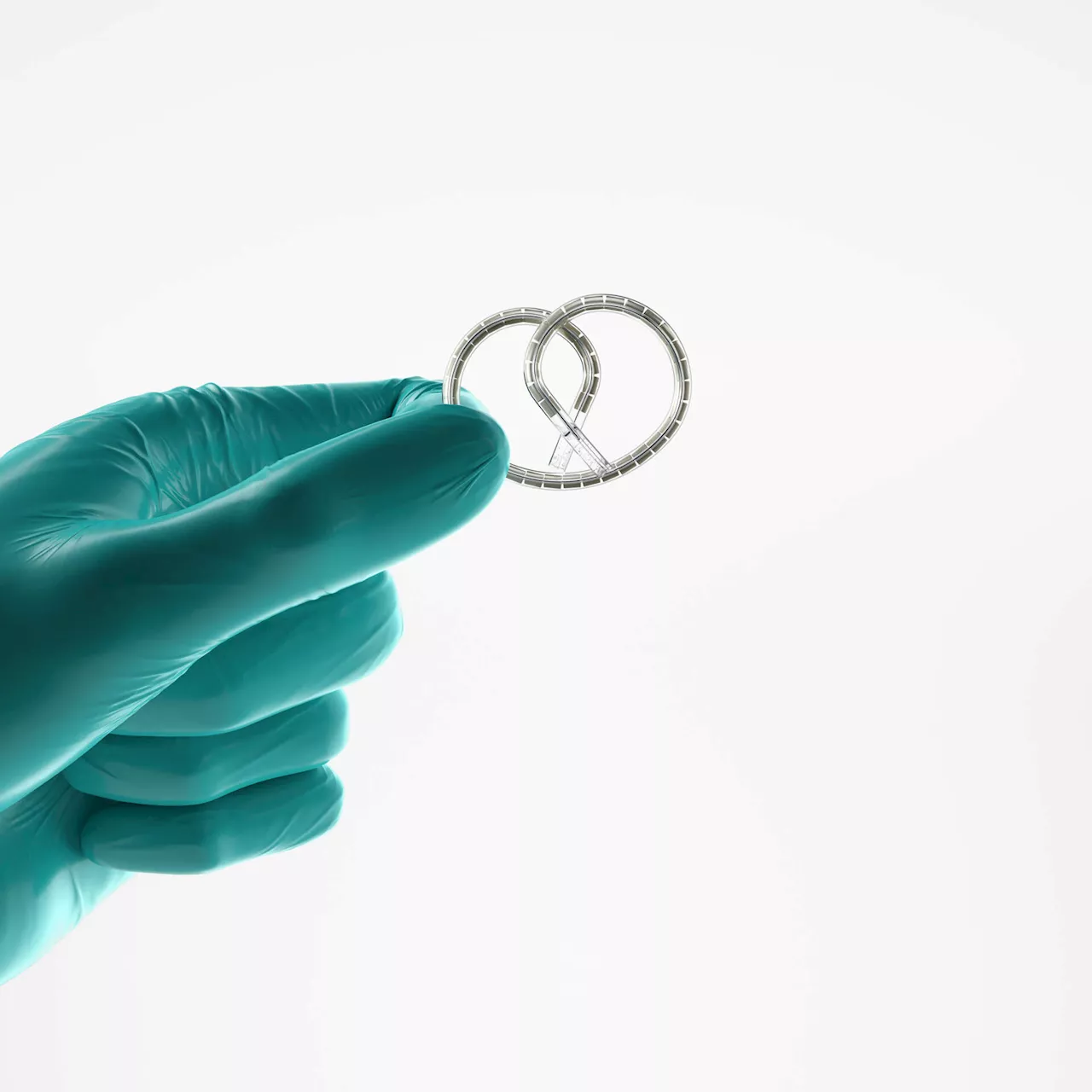
A groundbreaking treatment for bladder cancer has recently received approval from the U.S. Food and Drug Administration (FDA). The device, known as **Inlexzo**, is a pretzel-shaped implant designed to help patients retain their bladders while receiving cancer treatment. This marks a significant advancement for those diagnosed with the most common form of bladder cancer, which has seen no substantial innovations in over **50 years**.
Developed by **Dr. Chris Cutie**, a former urologist at **Mass General Brigham**, the Inlexzo device is engineered to slowly release cancer-fighting medications directly into the bladder. According to **Johnson & Johnson**, the company responsible for its development, preliminary trials have shown that **82%** of patients using the Inlexzo implant, in conjunction with medication, experienced complete disappearance of their cancer, while more than half maintained a cancer-free status after at least one year.
Historically, patients whose bladder cancer did not respond to chemotherapy faced a grim prognosis, often resulting in a **cystectomy**, a surgical procedure that removes part or all of the bladder. This operation can lead to significant lifestyle changes, including the use of an external urinary pouch. Dr. Cutie reflected on his previous role in performing these surgeries, stating, “It’s a very onerous operation.”
The challenge in treating bladder cancer has long been the difficulty of maintaining medications in the bladder. Dr. Cutie explained that the bladder’s natural function is to expel substances quickly, typically within **30 minutes** of drug injection. To combat this, doctors often instructed patients to hold their bladder for as long as possible after treatment. This uncomfortable method of prolonging drug exposure was largely ineffective, leading to the exploration of more innovative solutions.
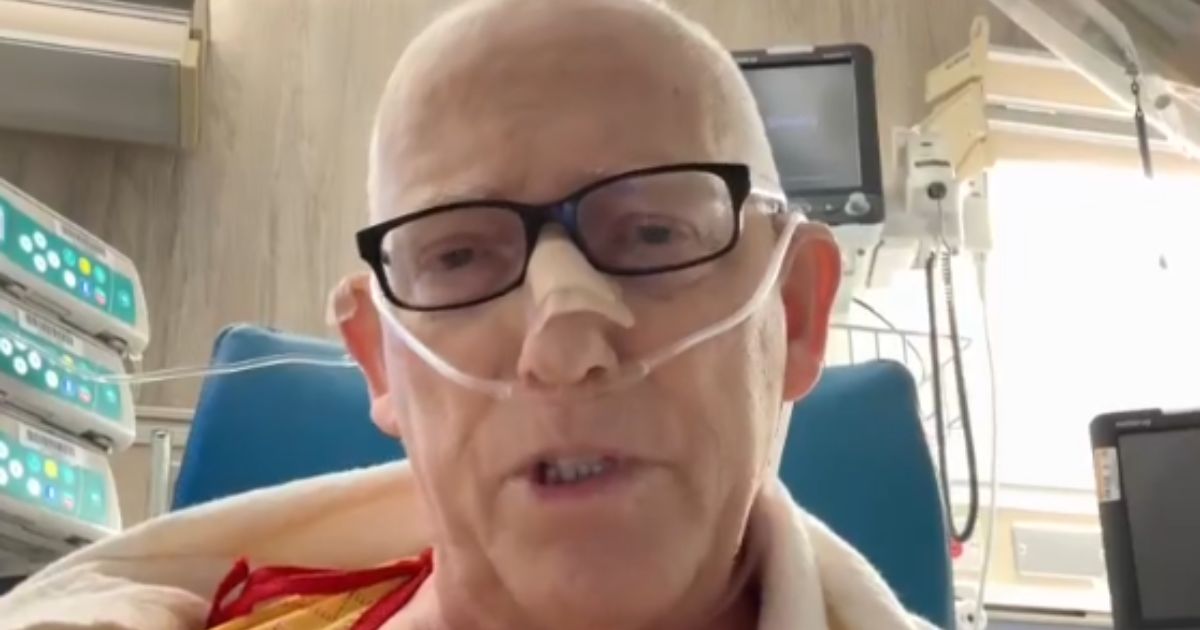
The introduction of the Inlexzo device could change the treatment landscape for bladder cancer. During clinical trials, patients with the device returned every three months for medication refills. While some reported mild irritation, many, like patient **Bill Parisi**, found the benefits outweighed the discomfort. Diagnosed in **2021**, Parisi had been facing the prospect of major surgery when his doctor recommended he join the clinical trial for Inlexzo. After **18 months** of treatment, he was declared cancer-free, and the device was subsequently removed.
Parisi expressed his relief, noting, “If you take my bladder out, that’s it. It would curtail my business.” His successful experience highlights the potential of Inlexzo to preserve patients’ quality of life while effectively treating their cancer.
Despite the promising results, experts urge caution regarding the long-term effectiveness of the device. **Dr. Adam Kibel**, chair of urology at Mass General Brigham, acknowledged the encouraging trial outcomes but stressed the need for further research to determine the overall impact on patient survival and quality of life. “This does a better job” of prolonging the exposure to cancer-fighting drugs, he said, although the insertion and removal process could be a consideration for patients.
As the medical community continues to explore innovative treatments, the Inlexzo device stands as a beacon of hope for bladder cancer patients, potentially transforming their treatment experience and outcomes.