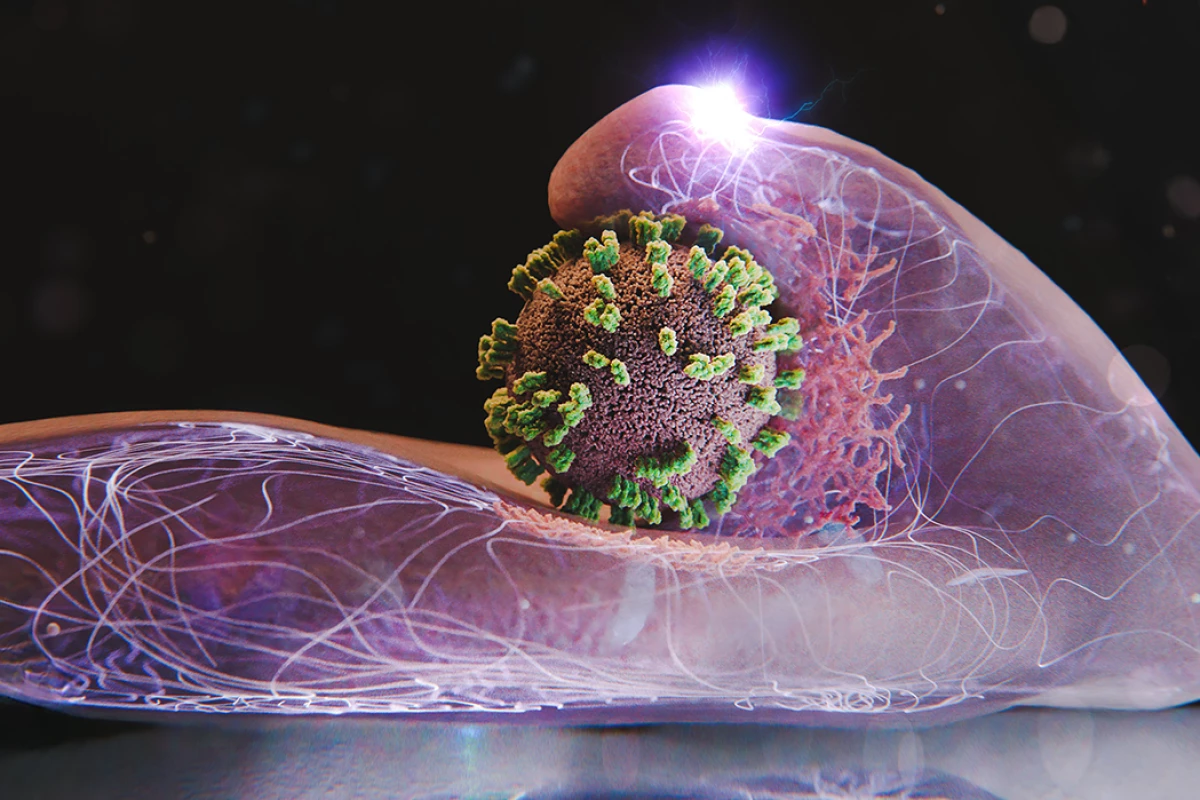
Research teams from Switzerland and Japan have made a significant breakthrough in understanding how the influenza virus infiltrates human cells. By developing a new imaging technique known as virus-view dual confocal and atomic force microscopy (ViViD-AFM), scientists can now observe the intricate process of viral entry in real time. This advancement sheds light on the mechanisms of infection that contribute to the seasonal spread of influenza.
The influenza virus relies on two critical proteins, hemagglutinin (HA) and neuraminidase (NA), to access host cells. These proteins act as molecular “keys” that bind to sialic acids on the surface of cells, enabling the virus to enter through a process called endocytosis. Traditionally, observing this rapid and minute interaction has presented challenges, as standard microscopes lack the resolution needed to capture such fleeting moments.
Breakthrough Imaging Technique Reveals Cellular Interaction
The newly developed ViViD-AFM allows researchers to visualize living human cells with unprecedented clarity. This hybrid microscopy method combines the strengths of atomic force microscopy and fluorescence microscopy, providing a detailed view of how the influenza virus engages with its target cells. For the first time, scientists have witnessed the nanoscale dynamics of viral invasion, revealing that cells do not passively succumb to infection. Instead, they actively respond, shifting and stretching as they interact with the virus.
According to Yohei Yamauchi from ETH Zurich, the interaction resembles a complex dance between the virus and the host cell. The team observed the behavior of individual flu virus particles as they navigated the cell surface under various conditions, such as when specific viral proteins were inhibited or when fewer binding sites were available.
The research also highlighted the role of the cell’s membrane in shaping the entry process. The scientists discovered that the flu virus requires the formation of larger bulges on the cell’s surface, driven by actin—a protein crucial for maintaining cellular structure. These bulges facilitate the virus’s entry, as the cell wraps it in a clathrin coat, ultimately pulling the virus inward and enclosing it within a vesicle.
Implications for Future Research and Medicine
The implications of this research extend beyond basic science; the ViViD-AFM technique could influence the development of antiviral therapies. By enabling researchers to observe how antiviral drugs function in real time within living cells, this tool holds promise for testing new treatments against the flu and potentially other viral infections.
Additionally, the versatility of ViViD-AFM could allow for the exploration of various cell-virus interactions, including the behavior of different strains of influenza and the mechanisms of vaccine efficacy. This innovative approach provides a new “window” into cellular processes, potentially revolutionizing our understanding of how viruses operate and how cells respond.
The findings from this groundbreaking study were published in the journal PNAS, marking a significant step forward in microscopy and virology research. As scientists continue to unravel the complexities of viral infections, this technology may pave the way for major advancements in biology and drug discovery.