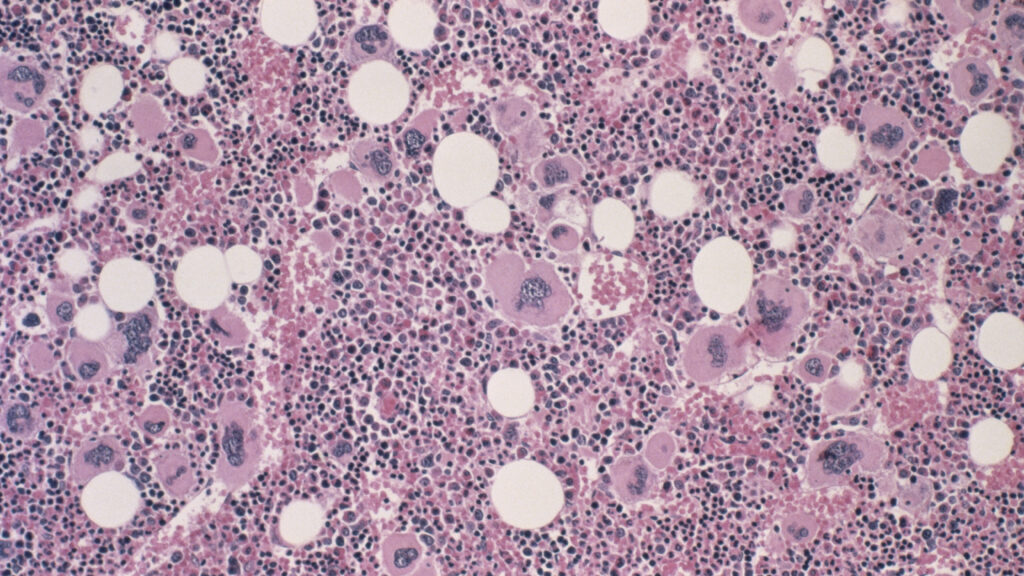
An experimental drug developed by Incyte has demonstrated significant spleen response rates and improvements in disease symptoms for patients suffering from advanced myelofibrosis, a type of bone marrow cancer. The results were presented during a conference in Orlando, Florida, signaling a potential shift in treatment options for this challenging condition.
The study findings indicate that the new drug targets a mutation in a protein known as calreticulin, which plays a critical role in the pathology of myelofibrosis. These preliminary results are expected to enhance Incyte’s development efforts for this innovative therapy, particularly as the company seeks to diversify its portfolio in light of impending patent expirations.
Shifting Market Dynamics
Incyte’s current flagship treatment, Jakafi, has been a major player in the myelofibrosis market, with projected sales reaching $3.5 billion in 2023. However, the company faces a pivotal challenge as the patent for Jakafi is set to expire in 2028. This looming expiration heightens the urgency for Incyte to introduce new therapies that can maintain its market position and continue to provide effective treatment options for patients.
Myelofibrosis is characterized by abnormal blood cell production in the bone marrow, leading to symptoms such as anemia, fatigue, and spleen enlargement. Current treatment options are limited, making the development of new therapies crucial for improving patient outcomes. The promising results from this study could offer hope to those affected by this debilitating disease.
Next Steps in Development
As Incyte moves forward, the company will need to conduct further clinical trials to solidify the efficacy and safety of the new drug. Continued investment in research and development is essential to ensure that the therapy can transition from experimental stages to widespread clinical use.
The results from the Orlando conference are just the beginning of what could be a transformative journey for Incyte and myelofibrosis treatment. Should the drug receive regulatory approval, it could significantly alter the landscape of treatment options available to patients, providing a new avenue for those who currently have limited choices.
In summary, Incyte’s research represents a pivotal moment in the fight against myelofibrosis. With a focus on innovative therapies, the company aims to not only sustain its market presence but also to enhance the quality of life for patients battling this complex disease.