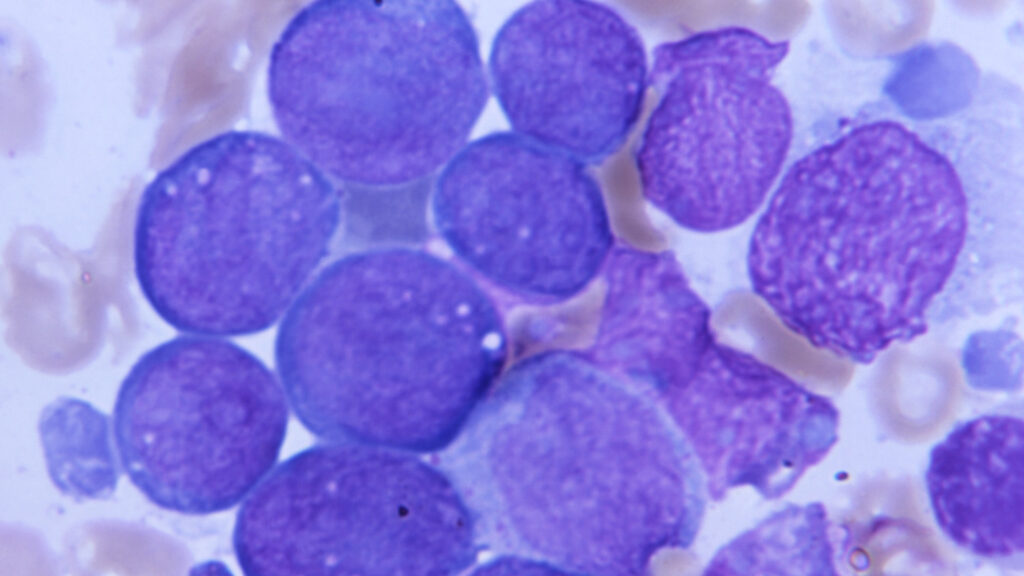
Terns Pharmaceuticals has unveiled promising data regarding its targeted drug for chronic myeloid leukemia (CML), potentially positioning it as a significant competitor to Novartis’ established therapies. The update was shared during the American Society of Hematology (ASH) annual meeting, which took place in December 2023 in San Diego.
The reported results highlighted that Terns’ drug not only maintained but also improved high molecular response rates among patients with advanced-stage CML. This finding could suggest a shift in treatment paradigms for this challenging condition, which affects thousands globally.
Key Findings from ASH Presentation
During the presentation, Terns Pharmaceuticals provided detailed insights into the clinical trial results, demonstrating a robust efficacy profile for its new drug. The company emphasized that the treatment achieved a high molecular response rate of over 70%, indicating its potential to outperform existing therapies available in the market.
According to Adam Feuerstein from STAT, the data presented at ASH positions Terns as a serious contender against Novartis, which has dominated the CML treatment landscape with its blockbuster drug. The impressive results could pave the way for Terns to capture a significant share of the market, especially among patients seeking alternatives to Novartis’ offerings.
The significance of these findings cannot be understated. Chronic myeloid leukemia, a type of cancer that affects the blood and bone marrow, has been traditionally managed with therapies that target specific genetic mutations. Terns’ approach appears to offer a new mechanism of action, potentially broadening treatment options for patients with this challenging disease.
Market Implications and Future Outlook
The competitive landscape for CML treatments is rapidly evolving. With Terns Pharmaceuticals now entering the fray, Novartis may face increased pressure to innovate and enhance their product offerings. The introduction of a successful competitor could lead to price adjustments and improved patient access to therapies, benefiting those affected by this condition.
As Terns prepares for potential regulatory submissions, the company will need to navigate the complexities of bringing a new drug to market. Securing additional clinical trial data and demonstrating long-term efficacy and safety will be crucial steps in this process.
The excitement surrounding Terns’ drug at the ASH meeting reflects a broader trend in oncology, where innovative therapies are increasingly being developed to address unmet medical needs. As more companies invest in research and development, patients with chronic conditions like CML may find themselves with enhanced treatment options in the future.
In conclusion, the developments presented by Terns Pharmaceuticals at the ASH annual meeting signal a potential shift in the treatment landscape for chronic myeloid leukemia. With promising data and a competitive edge, Terns is poised to challenge established players like Novartis, ultimately aiming to improve patient outcomes in this critical area of healthcare.